

















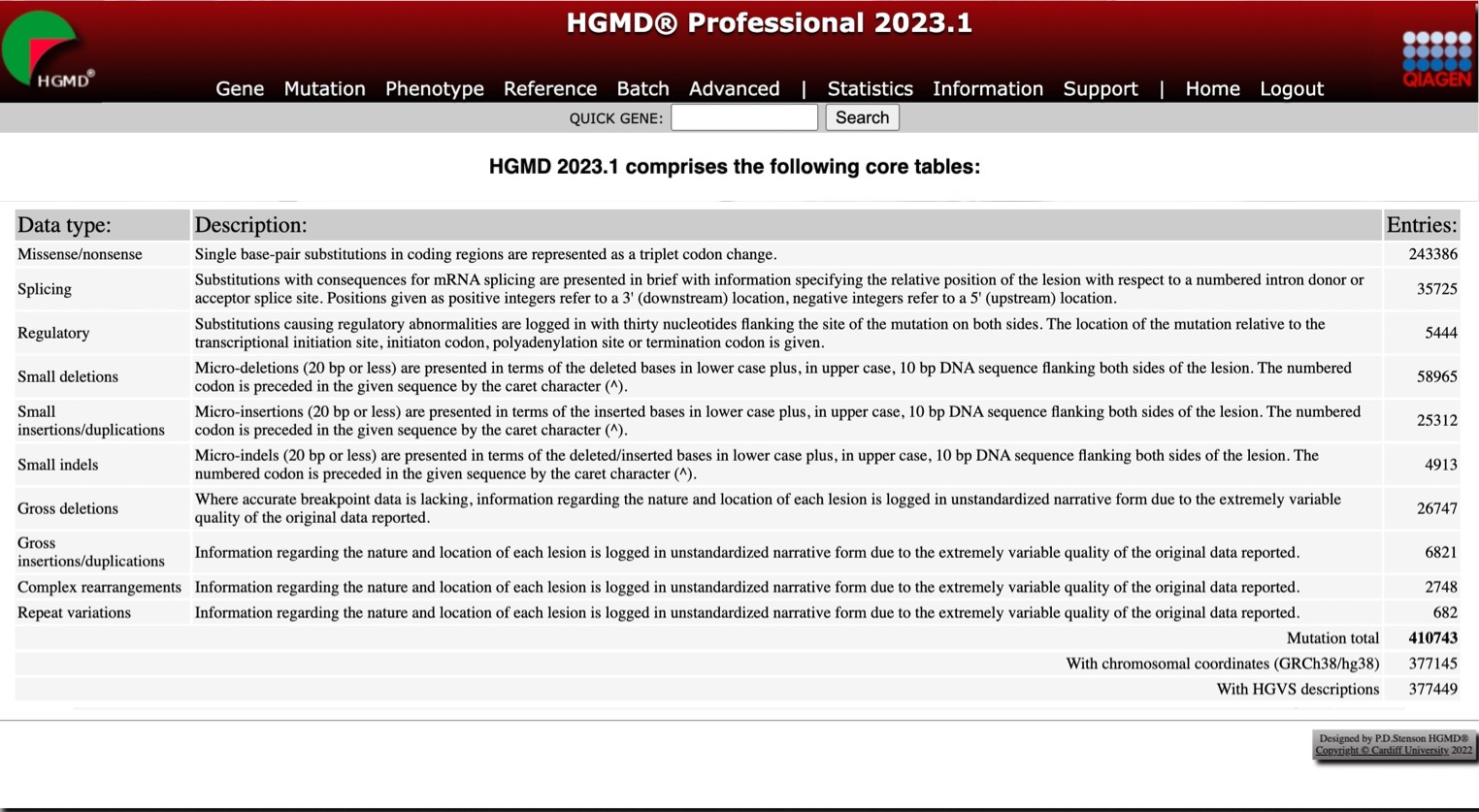
HGMD Professional 2023.1 is now available, expanding the world’s largest collection of human inherited disease mutations to 410,743 entries—that’s 12,102 more than the previous release.
For over 30 years, HGMD Professional has been used worldwide by researchers, clinicians, diagnostic laboratories and genetic counselors as an essential tool for the annotation of next-generation sequencing (NGS) data in routine clinical and translational research. Founded and maintained by the Institute of Medical Genetics at Cardiff University, HGMD Professional provides users with a unique resource containing expert-curated mutations all backed by peer-reviewed publications where there is evidence of clinical impact.
HGMD Professional is powered by a team of expert curators at Cardiff University. Data are collected weekly by a combination of manual and computerized search procedures. In excess of 250 journals are scanned for articles describing germline mutations causing human genetic disease. The required data are extracted from the original articles and augmented with the necessary supporting data.
The number of disease-associated germline mutations published per year has more than doubled in the past decade (Figure 1). As rare and novel genetic mutations continue to be uncovered, having access to the latest scientific evidence is critical for timely interpretations of NGS data.

Figure 1. Mutation entries in HGMD Professional 2023.1. The number of inherited disease-associated germline mutations published per year has more than doubled since 2010 (within 10 years).
View the complete HGMD Professional 2023.1 statistics, below.

Unlike new machine learning or artificial intelligence platforms that rapidly index millions of journal articles for mutations, HGMD Professional leverages human judgement and expertise—every catalogued mutation has been “touched” by a trained scientist to ensure accuracy, relevance, and context.
Learn more about the industry-leading database here, where you can explore features, watch videos, and request a complimentary 5-day trial.