

















When planning a family, information is powerful. QIAGEN Digital Insights is helping couples be informed and prepared.

Each time two carriers of the cystic fibrosis conceive, there is a 25 percent chance of passing the disease to their children; a 50 percent chance that the child will be a carrier of the cystic fibrosis gene; and a 25 percent chance that the child will be a non-carrier [1].
Recent developments in laboratory technologies have led to the commercial availability of expanded carrier screening panels capable of assessing hundreds of mutations associated with genetic diseases. Expanded carrier screening panels have the ability to identify mutations that would otherwise not be detected.


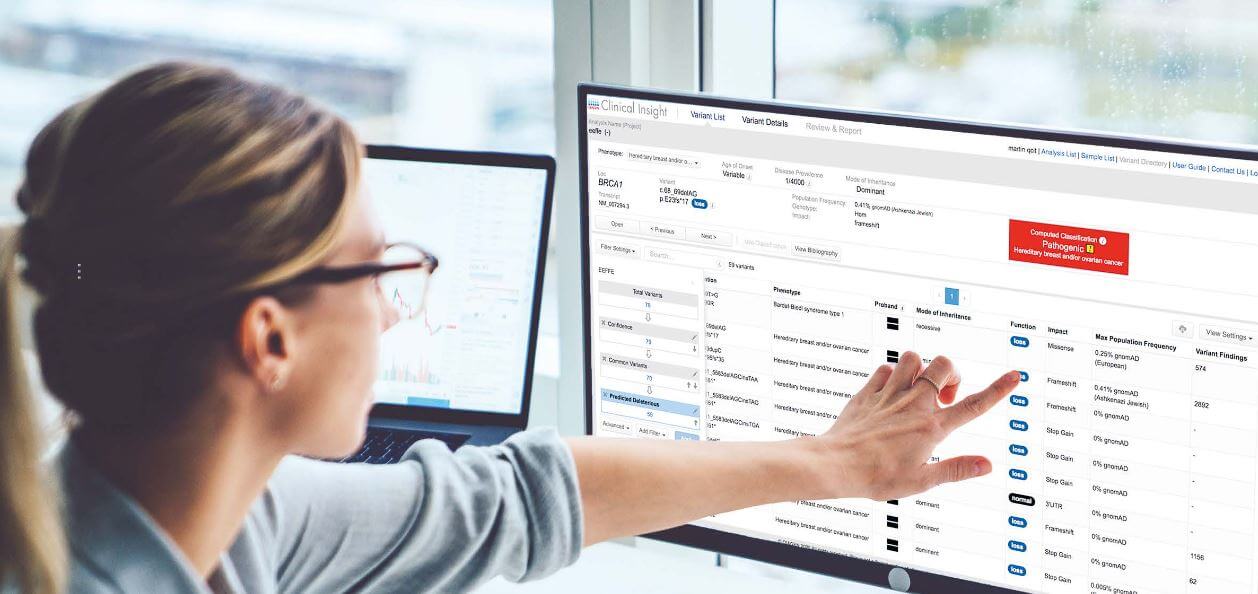

Learn more about QIAGEN's QIAseq Expanded Carrier Screening Panel.
References
Product Disclaimer:
QCI Interpret is an evidence-based decision support software intended as an aid in the interpretation of variants observed in genomic next-generation sequencing data. The software evaluates genomic variants in the context of published biomedical literature, professional association guidelines, publicly available databases, annotations, drug labels, and clinical trials. Based on this evaluation, the software proposes a classification and bibliographic references to aid in the interpretation of observed variants. The software is NOT intended as a primary diagnostic tool by physicians or to be used as a substitute for professional healthcare advice. Each laboratory is responsible for ensuring compliance with applicable international, national, and local clinical laboratory regulations and other specific accreditations requirements.