

















ON-DEMAND WEBINAR: Learn more about QIAGEN PGXI in an expert webinar. WATCH HERE
Each year, more than 134 million patients experience adverse drug events,
causing 2.4 million deaths worldwide (1).
For labs and pharmaceutical companies needing an easier and faster way to translate complex pharmacogenomic data into evidence-backed insights to inform drug delivery and development, QIAGEN PGXI provides a better a way forward.
An expert-curated pharmacogenomic knowledgebase providing comprehensive access to verified and up-to-date evidence on gene-drug associations to personalize drug therapies.
A PGx knowledgebase where scientific and translational researchers retrieve insights into gene-drug associations, conditions, and curated literature evidence for submitted inputs.
Unlike manual or semi-automated approaches to PGx annotation that can take hours or days, QIAGEN PGXI reduces turnaround time to minutes and eliminates the high cost of maintaining bioinformatic and technical support.

PGXI is a new PGx knowledgebase solution built upon a legacy platform used to deliver more than 1.6 million PGx annotations for more than 250 clinical research laboratories.
A proven solution that enables labs and pharmaceutical companies to efficiently and confidently produce evidence-backed insights for PGx markers and better understand implications for relevant medications, PGXI easily integrates into existing or new workflows to provide automated annotation of PGx data from all platforms, including NGS and arrays. Within minutes, users can retrieve a custom output of relevant information, including:
PGXI contains expert-curated and -verified data from all established PGx information sources, including:
PGXI provides automated annotation of PGx results. The solution:
Technology-agnostic, PGXI integrates seamlessly into your internal pipelines and workflows, allowing you to leverage cutting-edge pharmacogenomic content without disrupting your processes.
You access the data how you need it – whether through an application programming interface (API), flat files, or as data slices as a service.
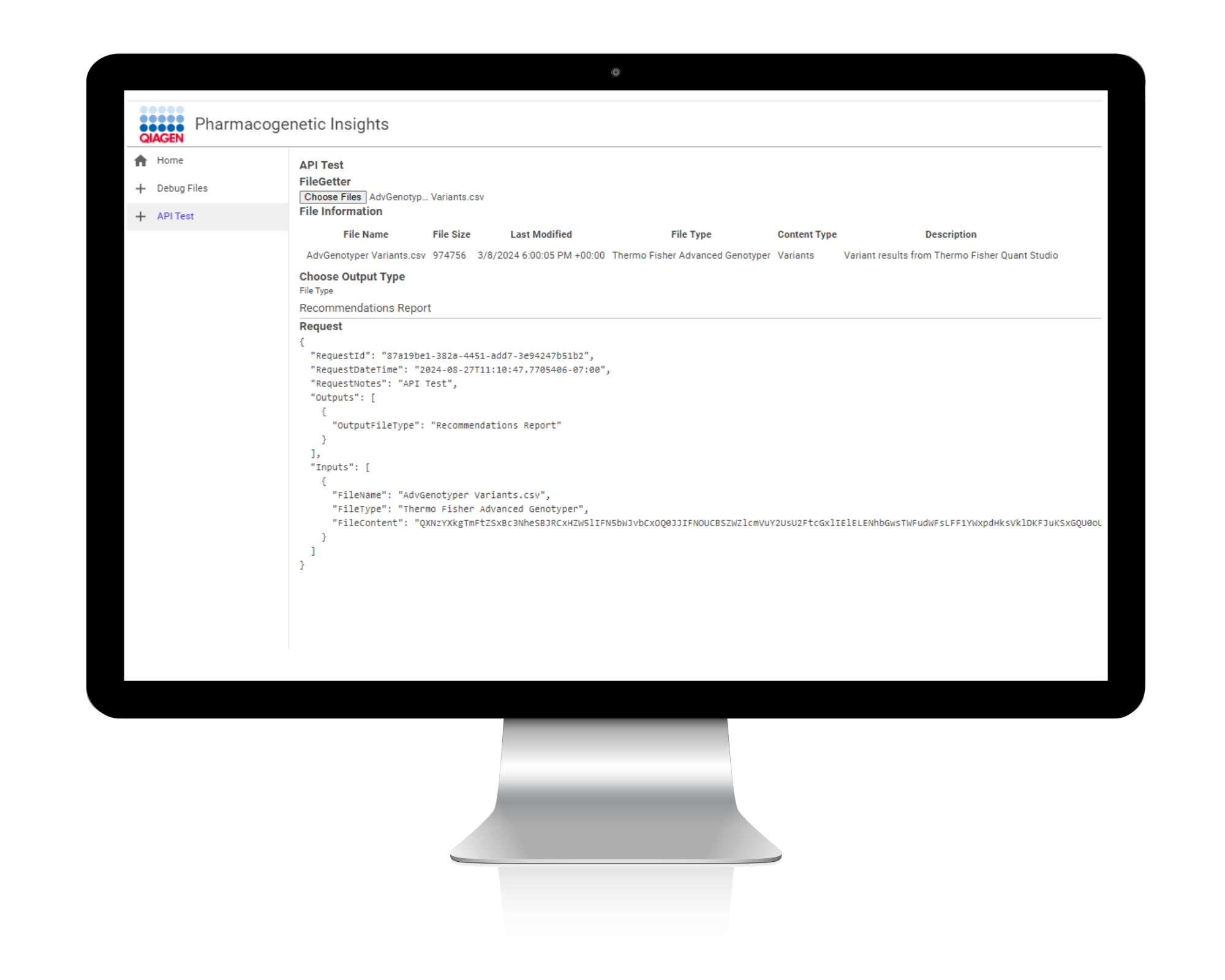
Rapidly query and identify associations linked to genotypes
By automating the identification of associations linked with genotypes, PGXI enables labs and pharmaceutical companies to efficiently and consistently identify medications associated with adverse effects.
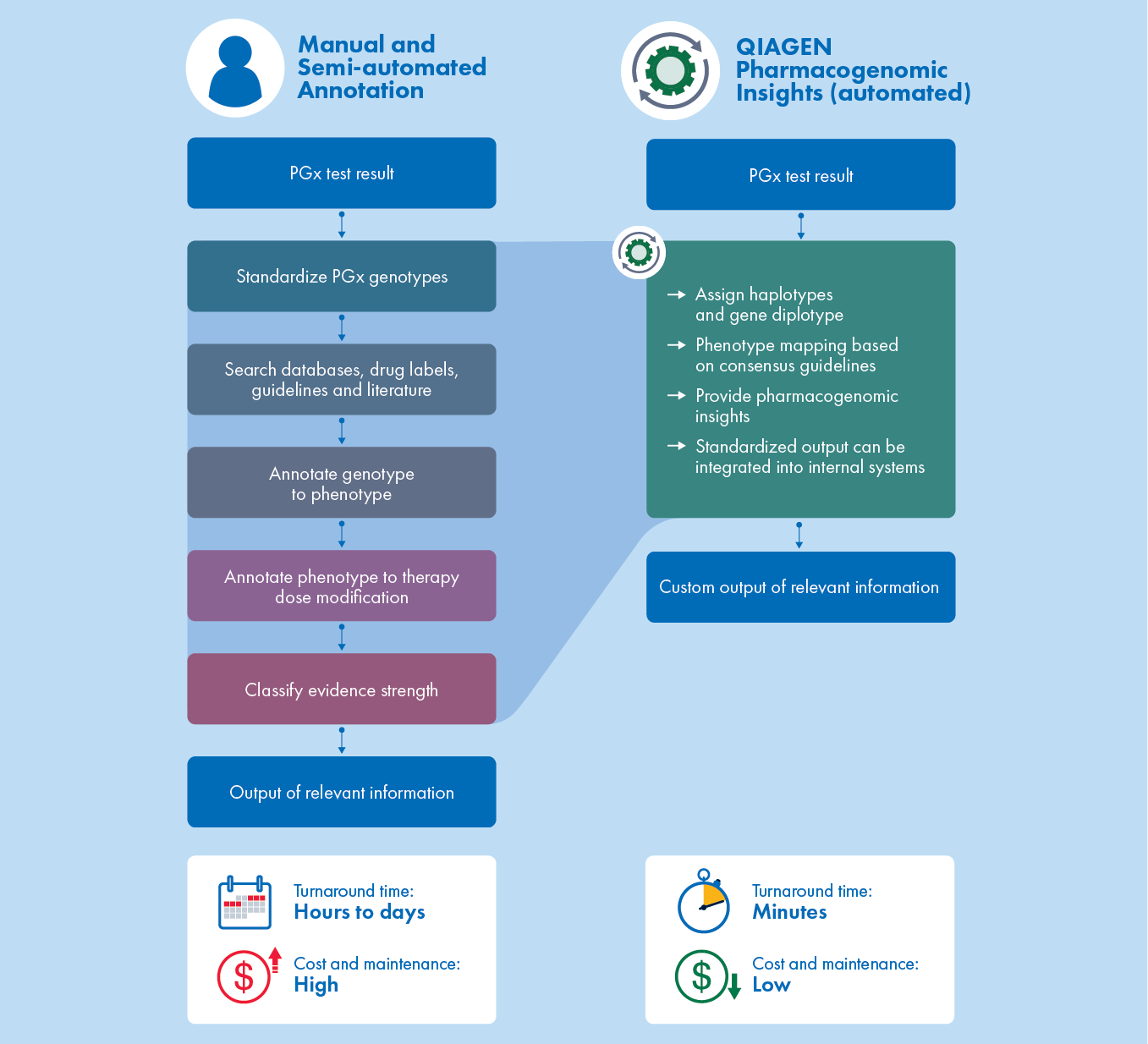
The most comprehensive pharmacogenomics content in the industry
PGXI leverages over a decade of experience in manually curating consolidated content from standard PGx sources, such as the FDA, CPIC, DPWG, PharmVar, PubMed, and more.
With the ability to rapidly assess PGx variants with expert-verified, quality content, labs and pharmaceutical companies can deliver trusted insights that can help improve outcomes and minimize overall health burdens.
Technology-agnostic integration with flexible access to comprehensive PGx content
PGXI is a technology-agnostic knowledgebase that ensures compatibility and flexibility across diverse platforms, including NGS and arrays.
Organizations can integrate and transition between different technologies and platforms without disruption, protecting their investments in existing infrastructure.
For labs and pharmaceutical companies, there is an easier and faster way to translate pharmacogenomic data into insights.
Discover how PGXI can transform your PGx program.
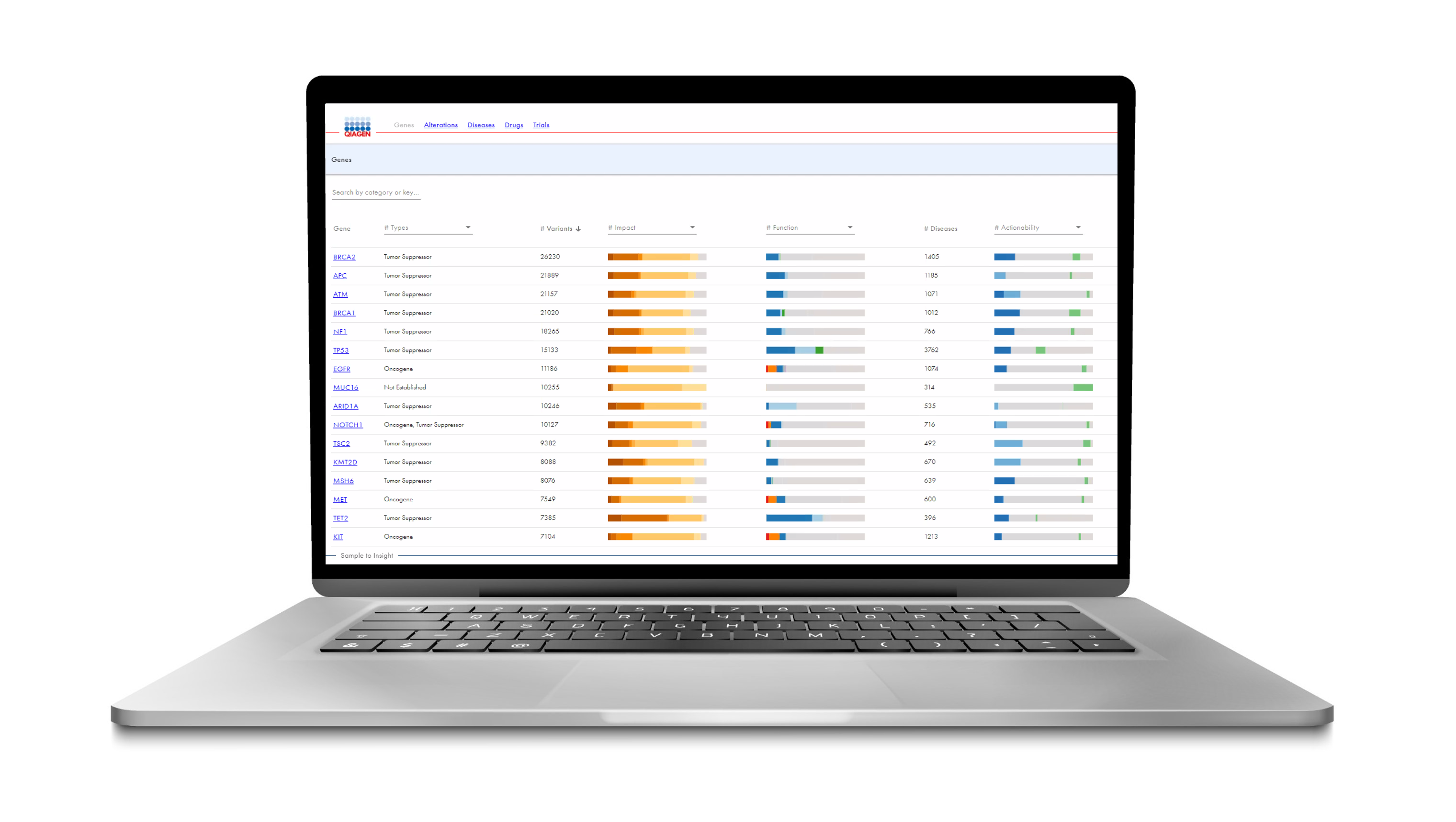


References:
1. Kamath A, Acharya SD, Poovizhi Bharathi R. Burden of death and disability due to adverse effects of medical treatment in India: An analysis using the global burden of disease 2019 study data. Heliyon. 2024;10(2):e24924-e24924. doi: https://doi.org/10.1016