

















When it comes to understanding life-changing cancer diagnoses, you can only trust the experience and expertise of QIAGEN
For clinicians, researchers, and pharmaceutical companies committed to advancing the treatment, management, and curability of cancer, would you trust AI, or would you trust data expertly curated by your peers and vetted by practicing, board-certified oncologists?
The Human Somatic Mutation Database (HSMD) is the only resource offering over two decades of manual variant curation and oncologist-reviewed evidence from over 600,000 real-world cancer patient cases. If you need a faster, simpler and confident way to understand and interpret the clinical significance of somatic variants, you need HSMD.
A pay-for-subscription resource for clinicians and diagnostic labs who need to enrich their somatic NGS interpretation pipelines with trusted somatic mutation data from over 1,400 cancer genes.
Comprehensive content - Includes the full database of gene mutations, offering the most up-to-date and complete collection of somatic mutations.
Advanced search tools - Allows for more sophisticated queries, enabling users to explore specific mutations, genes, or diseases in greater depth.
Requires a license - For clinical labs interpreting somatic mutations that impact patient care, HSMD requires a subscription license.
A free-to-access resource for researchers and academic institutions who want to explore the content of HSMD with access to data derived from 27 of the most clinically significant somatic genes.
Limited content - Provides access to a subset of the data available in HSMD Professional, consisting of only XXXXX.
XXXX - XXXXX
Free to access - HSMD Research is free to access for eligible research and academic users
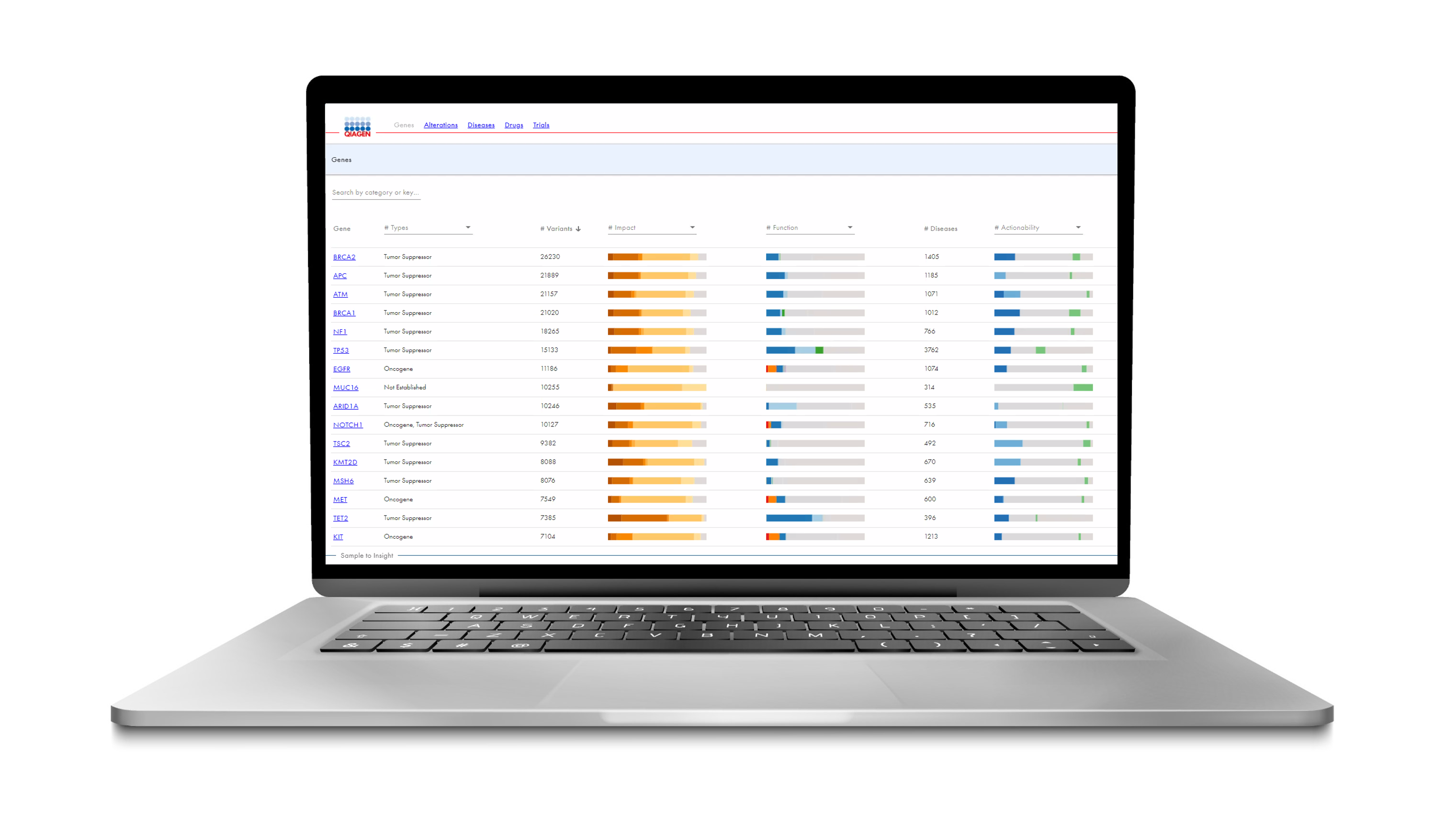
The Human Somatic Mutation Database (HSMD) is a new somatic database developed by QIAGEN that contains extensive genomic content relevant to solid tumors and hematological malignancies.
In the latest version of HSMD, the resource focuses on providing deep insight into small variants, such as SNVs, indels, frameshifts, fusions and copy number variants that have been clinically observed or curated from scientific literature to help users better understand and define precise function and actionability.
Available as a web-based application, this expert-curated resource contains content from over 547,000 real-world clinical oncology cases combined with content from the QIAGEN Knowledge Base (QKB), providing gene-level, alteration-level, and disease-level information.

When a variant has been “clinically observed,” it means our professional clinical interpretation service has encountered this alteration in a real-world clinical case.
For these variants, our team has assessed the clinical and biological relevance and calculated the gene and variant prevalence across observed tumor types.
Conversely, content from the QKB is proactively curated from scientific literature; therefore, not all variants have yet been directly clinically observed by our professional clinical interpretation services.
HSMD leverages expert-curated content from the QIAGEN Knowledge Base, which contains the latest evidence from peer-reviewed papers, clinical and functional studies, on- and off-label drugs, and professional guidelines, such as NCCN, AMP/ASCO/CAP and the World Health Organization (WHO), and commonly used databases, including gnomAD and ClinVar.
With this content, you gain insights into:


COSMIC, the Catalogue of Somatic Mutations in Cancer, is the world’s largest expert-curated somatic mutation database. HSMD complements COSMIC with data from real-world oncology cases to better understand gene and variant prevalence. HSMD also provides deeper variant annotations. Therefore, COSMIC is used to identify biomarkers and annotate variants for genomic reports, whereas HSMD is used to validate biomarkers and better assess their biological and clinical relevance.

Explore HSMD's features, content and applications with complimentary 5-day trial.