


















Targeted next-generation sequencing (NGS) is an ideal tool for studying the genetic basis of cancer, facilitating the survey of large numbers of targets (potential variants) in parallel, while also offering high sensitivity. QIAGEN’s new barrier-breaking Sample to Insight Oncology Solution combines targeted DNA and multimodal pan-cancer panels, one of the fastest and cheapest secondary analysis in the market and trusted variant interpretation and reporting software powered by augmented molecular intelligence.
Sample to Insight Workflow for Oncology
QIAseq Targeted DNA Pro and Multimodal Pan Cancer Panels set a new standard for NGS genotyping. Major innovations within this chemistry provide improved ease-of-use, efficiency and expanded variant analysis to simplify and speed-up your NGS workflows.
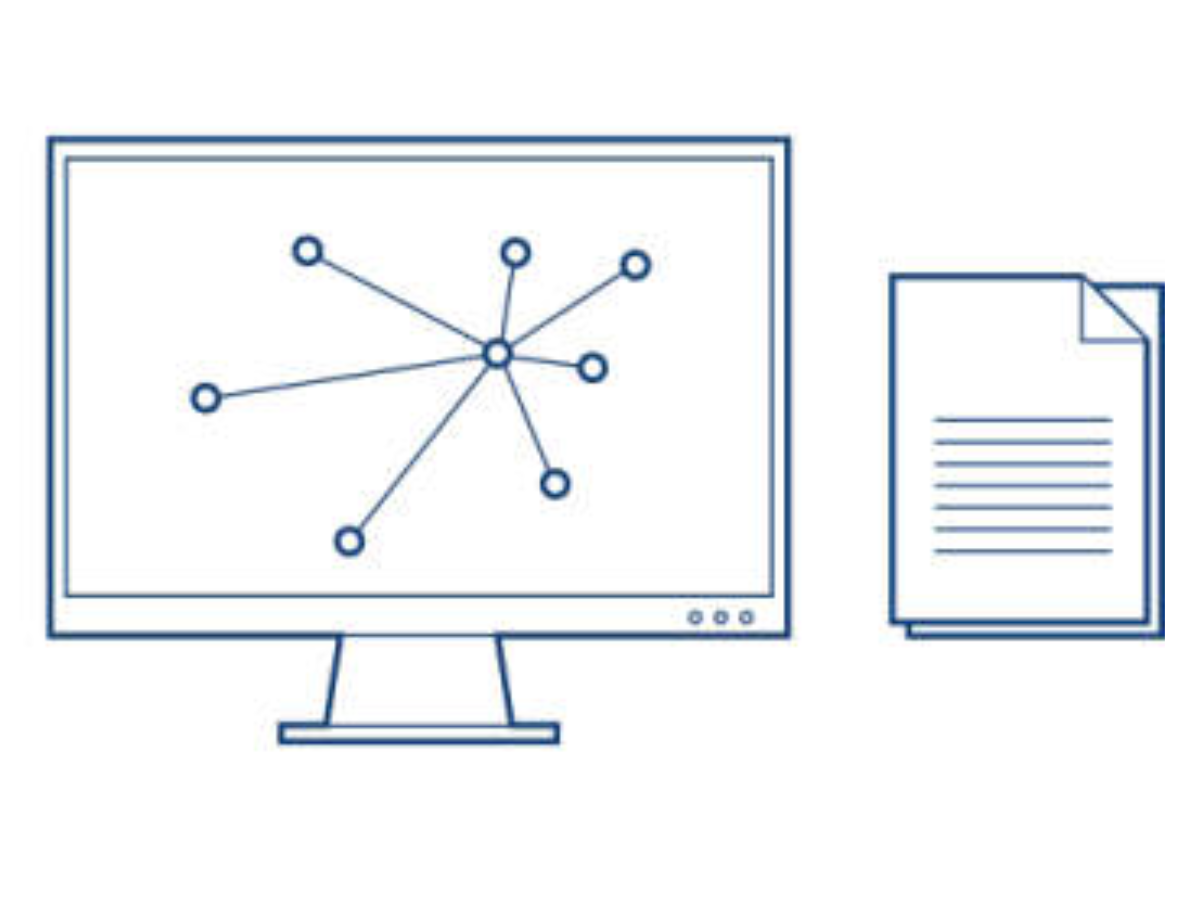
QCI Interpret is NGS variant interpretation and reporting software powered by augmented molecular intelligence that helps labs not only make faster decisions—but the right decisions.
Connected to the exclusive QIAGEN Knowledge Base, the industry’s most comprehensive, manually curated resource updated weekly, QCI Interpret delivers variant-specific, scientific evidence in context of phenotype or diagnosis. Interactive filters prioritize variants and proprietary algorithms transparently compute AMP/ASCO/CAP and ACMG/AMP variant classifications, enabling users to generate evidence-based reports with efficiency, confidence, and reproducibility.



Let us help you optimize your NGS analysis pipeline. Our services team is here to answer your questions and help you get from sample to final report in less time, for less money.