


















For tens of thousands of patients, precision medicine is rewriting their cancer stories.
Linda Boyed, for example, a 52-year-old occupational therapist, was on vacation with her family in Hawaii, when she noticed something was not right. She was becoming incredibly fatigued while swimming at the beach and taking long walks around her hotel. By the time she returned home to Columbus, Ohio, her skin and eyes had yellowed. Her doctor referred her to an oncologist who said the three words nobody wants to hear: you have cancer. But for Linda, the words had greater gravity. Her bile duct cancer had already spread too far for chemotherapy or surgery to be effective. Her oncologist offered to keep her comfortable for her final few months.
Boyed's husband refused to accept that prognosis. He found a doctor at the Ohio State Comprehensive Cancer Center who was conducting a clinical trial on an experimental drug for gastrointestinal cancers. Boyed enrolled in the study. Genetic tests on her tumors revealed a mutation in a gene called FGFR (short for "fibroblast growth factor receptor"), which was likely spurring the cancer's growth. As part of the trial, Boyed was given the experimental drug, called BGJ398 (infigratinib), to inhibit the action of the FGFR mutation. Boyed's symptoms cleared up, the tumors stopped growing, and she regained the weight she had lost (1).
That was four years ago.
Stories like Boyed's are being told across the United States. As new cancer drugs emerge from labs and enter clinical trials, the days when cancer patients received one-size-fits-all regimens of chemotherapy and radiation may soon be a thing of the past. Today, doctors are taking a far more nuanced and personalized approach. What is known as precision medicine, this new concept uses genetic tests—of both the patient and the cancer tumor—to determine the exact drugs or treatments that have the best chance of working.
Although precision-medicine techniques are available for many diseases, their impact is being felt most strongly in cancer treatment. Researchers are building a growing list of genes and genetic mutations that show up in tumors and matching them to drugs that can stop them. The cancer genes that drugs can target now number in the tens of dozens, and researchers are hot on the trail to discover hundreds more.

But even then, most patients don't get that one-in-ten chance. Many doctors still lack expertise in the area and fail to administer the genetic tests that could open the door to a precision medicine treatment. Expense is also an obstacle: Insurance companies don't reimburse adequately for the tests. For these reasons, only 10 percent of cancer patients undergo genetic testing. Precision medicine is helping, at best, only a few percent of the nearly 2 million people who are diagnosed with cancer in the U.S. each year, and the fraction is much smaller among the 17 million cancer patients worldwide (7).
To increase the number of patients eligible for treatment, doctors are turning to technology for help. Genetic testing is churning out so much data that even an army of PhDs can’t make sense of it all. Computers and algorithms are able to turn that volume of data from a liability to an advantage. Scientists are now delegating the task of finding the weaknesses in cancer tumors to clinical decision support software that can churn through millions of genetic test results and patient outcomes to find a relationship between tumor genes, cancer growth, and specific drugs.
To increase the odds that a cancer patient who walks through their doors is given a treatment option, City of Hope National Medical Center outside of Los Angeles plans within two years to be the first major hospital in the U.S. to do genomic testing on the tumors of every single one of its 9,000 cancer patients a year (1).
As other hospitals follow suit, they are generating vast amounts of data. The 20,000 genes of a typical human genome include three billion DNA nucleotides, any of which can be mutated, repeated or moved in any number of ways to cause cancer. Each of the human body's billions of cells has its own copy of the genome, subject to its own mutations.

But DNA is only part of the picture: Whereas DNA is a blueprint, the real work in our cells is carried out by proteins—complex molecules that control almost everything in our biology. Proteins govern both the growth of a cancer tumor and the work of the immune system in fighting it. There are as many as 6 million basic proteins and variations on them, and researchers are now measuring thousands of them directly in cancer-tissue samples and feeding that information to the deep-learning programs.
Computers don’t work the way scientists do—they never "understand" the biology behind the cancer they're analyzing. Instead, they digest reams of information from tissue samples of cancer patients, and correlate that information with documented evidence from research and clinical studies to help clinicians better understand if their patient will respond to a specific treatment.
Clinical decision support software can tease out patterns in the data that a human could never see—linking, for example, the presence of the FGFR gene to a particular cancer of the bile duct.
While human intelligence is far superior to computer intelligence, clinical decision support software can serve as a scaffold to human decision-making, much as computer programs assist pilots.
Thousands of different mutations in a patient's genome can shape the development of cancers and determine which treatments are effective. On top of that, each cancer cell is a moving target, continually developing new mutations that can help it evade immune cells and survive powerful cancer drugs. This means that for every cancer patient who undergoes genetic testing, tens of thousands of data points must be considered to reach the right diagnosis and best possible treatment pathway.
Clinical decision support software can help scientists analyze and classify data significantly faster. What used to take weeks, interpreting a large multi-gene cancer panel can now be done in a matter of minutes. This is life-changing for individuals with the deadliest, most aggressive cancers.

For example, glioblastoma (brain cancer) has the lowest median survival time from diagnosis—15 months—of any major cancer (8). The disease works so quickly that there’s not enough time to try multiple treatments in search of one that eventually works. Treating oncologists need to know right away which therapy has the best chance of working and put their patients on that care plan immediately.
Yet clinical decision support software does more than save time and lives. It also saves money. By looking for specific genetic signatures in each patient, clinical decision support software can help doctors know ahead of time whether or not a patient’s tumor will respond to a treatment, which can save the patient from unnecessary complications and expenses. In fact, a recent study found that clinical decision support software can reduce the cost of patient management by at least 35 percent (9). For most cancer patients, this equates to thousands of dollars.
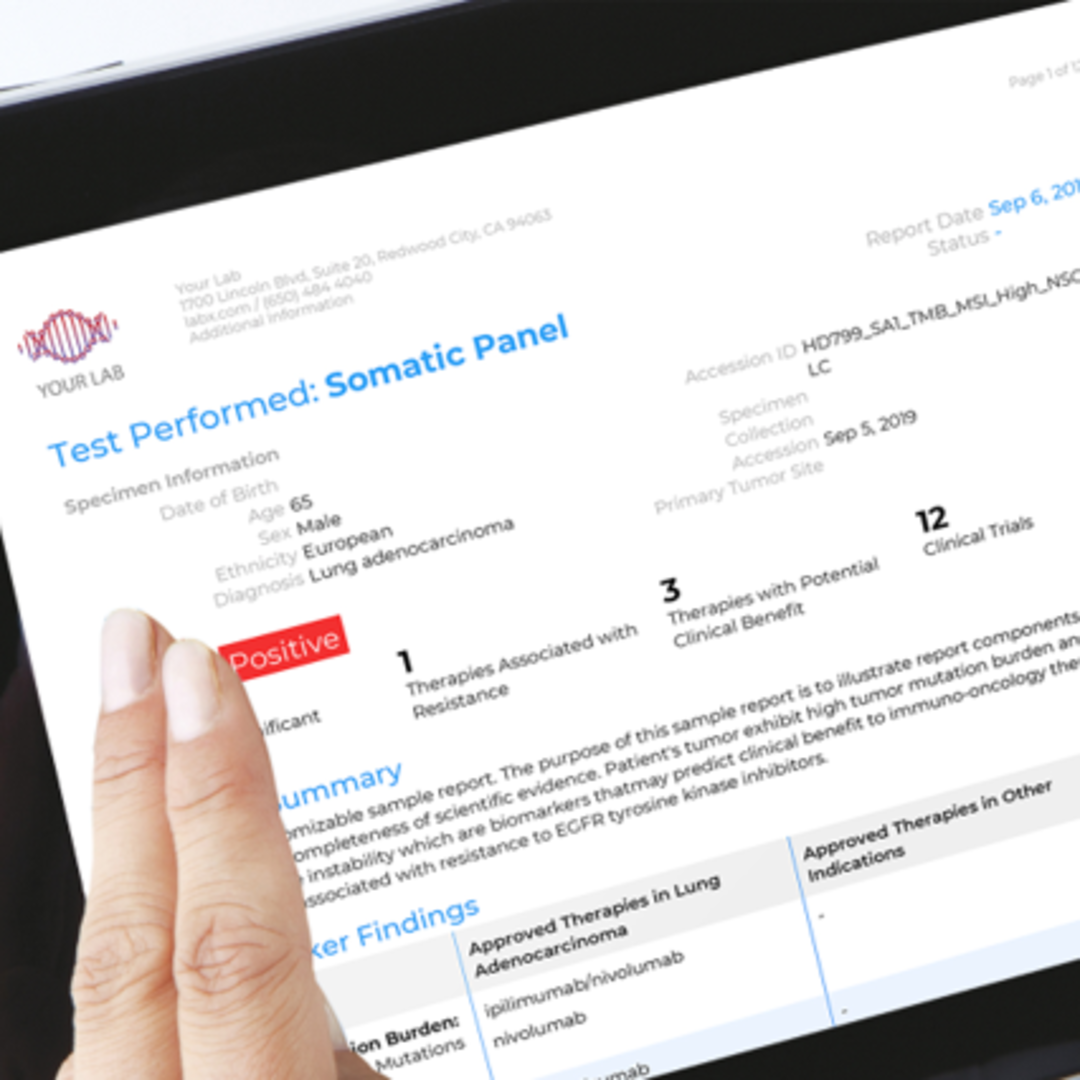
For example, QIAGEN, a world leader in molecular diagnostic testing, has developed clinical decision support software that can aid in the diagnosis and care of over 200 different types of cancer. Known as QIAGEN Clinical Insights (QCI), the software interprets a cancer patient’s genetic data, building a personalized report that informs the oncologist of which genetic mutations are likely causing the cancer, which precision therapies are known to work against these mutations, and where the patient can go to access these treatments or trials.
The significance of clinical decision support software in the advancement of cancer care cannot be overstated. According to a study in JCO Clinical Cancer Informatics, decision support systems are “one of the greatest potential benefits of a digital health care ecosystem” (11). The technology is enabling faster diagnoses, better patient outcomes, and saving patients and healthcare organizations tens of thousands of dollars in the process.
Freedman, David. “Precision Medicine Is Crushing Once-Untreatable Cancers.” Newsweek, 16 July 2019, www.newsweek.com/2019/07/26/targeting-each-patients-unique-tumor-precision-medicine-crushing-once-untreatable-cancers-1449287.html.
Aisner Dara et al. “Effect of expanded genomic testing in lung adenocarcinoma (LUCA) on survival benefit: The Lung Cancer Mutation Consortium II (LCMC II) experience.” Journal of Clinical Oncology. 34,15 (2016).
Zhao, Hongyun et al. “Final overall survival results from a phase III, randomized, placebo-controlled, parallel-group study of gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer.” Journal of Thoracic Oncology: Official publication of the International Association for the Study of Lung Cancer 10,4 (2015): 655-64.
Hida, Toyoaki et al. “Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial.” Lancet (London, England) 390,10089 (2017): 29-39.
Paik, Paul K et al. “Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping.” Cancer discovery 5,8 (2015): 842-9.
“Find Trials - ClinicalTrials.Gov.” National Institute of Health, 1 Dec. 2020, clinicaltrials.gov/ct2/search. Cancer Statistics
“Cancer Statistics.” National Cancer Institute, 25 Sept. 2020, www.cancer.gov/about-cancer/understanding/statistics.
The Brain Tumour Charity. “Glioblastoma Prognosis | Brain Tumour Survival Rates.” The Brain Tumour Charity, 12 Sept. 2020, thebraintumourcharity.org/brain-tumour-diagnosis-treatment/types-of-brain-tumour-adult/glioblastoma/glioblastoma-prognosis.
“How Information Technology Leaders Will Pave The Road To Precision Medicine.” Health IT Outcomes, 30 Apr. 2019, www.healthitoutcomes.com/doc/how-information-technology-leaders-will-pave-the-road-to-precision-medicine-0001.
Pawloski, Pamala A et al. “A Systematic Review of Clinical Decision Support Systems for Clinical Oncology Practice.” Journal of the National Comprehensive Cancer Network: JNCCN vol. 17,4 (2019): 331-338.
Aisner Dara et al. “Effect of expanded genomic testing in lung adenocarcinoma (LUCA) on survival benefit: The Lung Cancer Mutation Consortium II (LCMC II) experience.” Journal of Clinical Oncology. 34,15 (2016).