

















Next-generation sequencing (NGS) of circulating tumor DNA (ctDNA) supports blood-based genomic profiling but is not yet routinely implemented in the setting of a phase I trials clinic. TARGET is a molecular profiling program with the pri-mary aim to match patients with a broad range of advanced cancers to early phase clinical trials on the basis of analy-sis of both somatic mutations and copy number alterations (CNA) across a 641 cancer-associated-gene panel in a single ctDNA assay.
In April 2019, Cancer Research UK's TARGET (Tumor chARacterisation to Guide Experimental Targeted therapy) study added new evidence for the feasibility and potential utility of liquid biopsy to identify clinically actionable mutations and guide clinical trial enrollment for patients with advanced cancer.

In the first of the two-part trial, the investigators collected, processed, and analyzed blood samples from 100 patients. The results showed that a small volume of blood can contain up-to-date genetic information about a patient’s cancer to inform treatment choices. In this feasibility study of the first 100 patients, Cancer Research UK used QIAGEN Clinical Insight (QCI) to molecularly match and enroll 11 patients into a targeted therapy clinical trial.

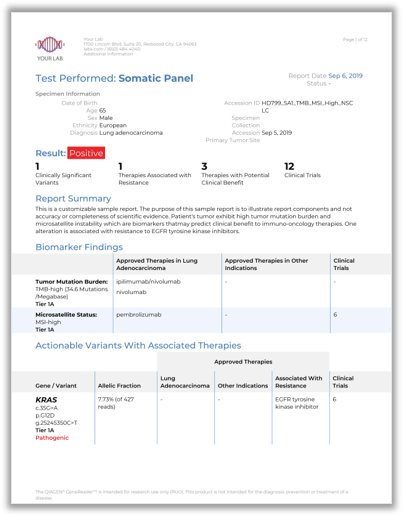
QCI is a cloud-based clinical decision support software platform used to generate actionable insights from next-generation sequencing (NGS) data.
QCI leverages QIAGEN’s expert manually curated evidence knowledge base that includes more than 20 million biomedical findings and is the world’s largest commercial database of curated evidence data on somatic and inherited genetic variants. QCI minimizes the complexity and cost of determining the significance of NGS data and automates guidelines for clinical actionability from leading oncology and pathology organizations.