

















AAV-based gene therapies are transforming disease treatment, but manufacturing challenges like heterogeneity, contamination and hidden mutations threaten safety, efficacy and cost. Even rare sequence errors can disrupt protein expression or packaging, putting the therapy at risk.
Regulators now recommend next-generation sequencing (NGS) over traditional assays for its sensitivity and unbiased insights. However, implementing NGS in GMP-compliant settings poses hurdles – from data gaps to limited digital solutions – amid mounting pressure to accelerate timelines.
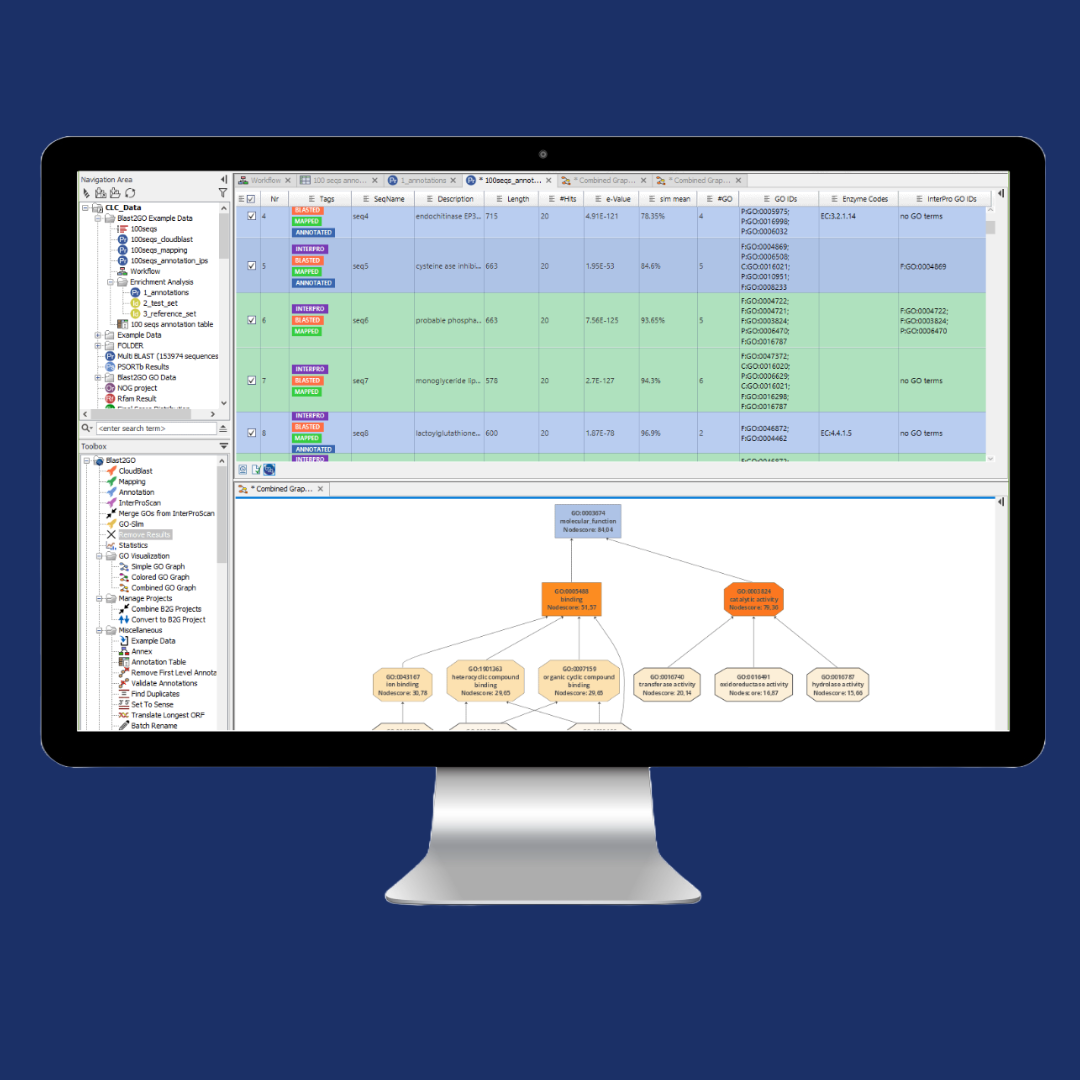
Implementing GMP-compliant NGS workflows requires more than sequencing – it demands validated bioinformatics pipelines and integrated access to product and process data for confident decision-making. By bringing analysis in-house with CLC Genomics Workbench, biopharma teams can streamline compliant, end-to-end workflows and collaborate more effectively. The result?



QIAGEN CLC Genomics Workbench is purpose-built to help biopharma organizations implement NGS-based assays within GMP frameworks – ensuring rigorous QC, data integrity, and safety standards. Designed for reliability and ease of use, it provides everything needed to detect viral contaminants and genetic instability with confidence.
Compatible with all major NGS technologies, including Illumina, 10x Genomics, Oxford Nanopore, due to flexible, modular and standards-based architecture.
Minimizes human error through automated data capture and secure, timestamped documentation—ensuring consistent, audit-ready compliance and full traceability in line with GMP requirements.
A secure server enables real-time error tracking, digital signatures and streamlined access, while role-based authentication protects sensitive data and restricts critical changes to authorized users.
Accurately detect low-frequency variants with validated algorithms and trace genetic changes across production stages using intuitive, auditable workflows tailored for viral vector and vaccine manufacturing.
Comprehensive workflows for RNA-seq, variant calling, metagenomics and single-cell analysis combine with a user-friendly interface, customizable pipelines, and pre-configured protocols to streamline advanced NGS with the latest algorithms.
Deploy on existing infrastructure or in the cloud to enable cost-efficient computing, while standardizing and scaling bioinformatics pipelines for consistent, compliant batch release and regulatory readiness.



See how the QIAGEN CLC Genomics Platform enables full control over operational system checks, optimized workflows and automated workflow execution.

Learn how to build and customize workflows using CLC Genomics Workbench for DNA-seq, RNA-seq, OTU clustering, de novo assembly and more.




1. Schweizer L. Drug Intelligence Science (DIS®): Pioneering a high-resolution translational platform to enhance the probability of success for drug discovery and development. Drug Discovery Today. 2023;28(11):103795-103795. doi:https://doi.org/10.1016/j.drudis.2023.103795