

















As our molecular knowledge of cancer continually evolves, a new era in cancer care is beginning: comprehensive genomic profiling (CGP). With a single CGP test, we can map an individual’s unique genomic profile across all 4 types of alterations known to drive cancer growth. Capable of interrogating hundreds of genes, CGP provides deeper insight to help oncologists determine the best possible treatment for each patient and chart a treatment journey to identify courses of action should the disease progress. Now, the question is how to perform CGP faster.
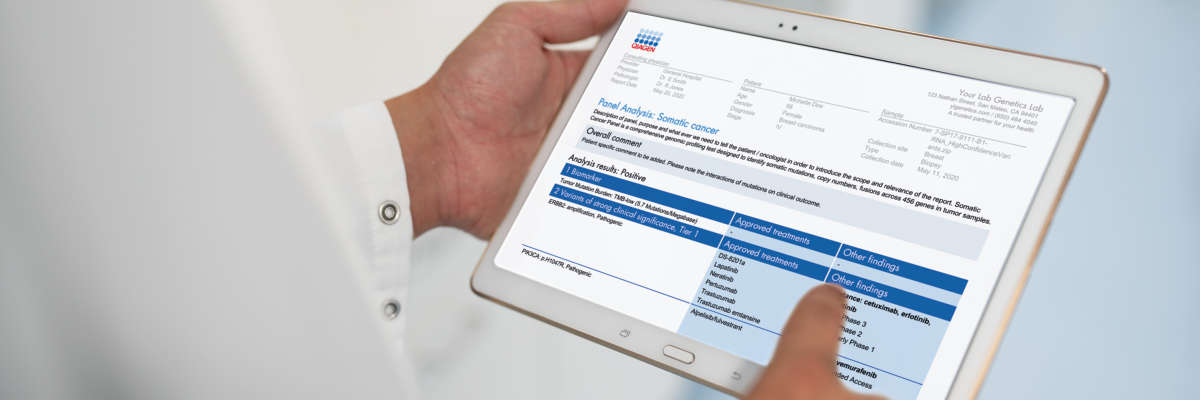
QCI Interpret for Oncology is an informatics platform powered by augmented molecular intelligence that enables rapid, evidence-powered comprehensive genomic profiling at scale.
For labs using the Illumina® TruSight Oncology 500 assay, QCI Interpret offers a preconfigured, FASTQ to final report workflow that can detect single nucleotide variants (SNVs), indels, gene fusions, RNA splice variants, copy number variants (CNVs), tumor mutational burden (TMB), and microsatellite instability (MSI).
Significantly improving productivity and test turnaround time, QCI Intepret's preconfigured workflow for TruSight Oncology 500 helps molecular diagnostic labs deliver actionable reports with speed, consistency, and confidence.
Trusted by labs around the world, QCI Interpret for Oncology processes over 15,000 cases each month. To date, the software's knowledge base has been used to interpret over 3 million patient cases.
Built over 25 years and updated weekly, the QIAGEN Knowledge Base leverages machine learning to rapidly index journal articles for mutations and human judgment and expertise (augmented molecular intelligence) to ensure accuracy, relevance and context—every catalogued “finding” has been “touched” by a trained scientist.
The content core of QCI Interpret for Oncology, the QIAGEN Knowledge Base is the world’s largest and
fastest growing collection of globally trusted molecular knowledge.
QCI Interpret for Oncology contains over 6.4 million precise variants characterized in 18,000 genes.
With over 5,000 new variants added each month, QCI Interpret for Oncology adds over 60,000 new variants per year.
Trusted by labs around the world, QCI Interpret for Oncology processes over 15,000 cases each month.
While offering preconfigured workflows for the Illumina TruSight Oncology 500 assay, QCI Secondary Analysis and QCI Interpret are panel- and workflow-agnostic and can utilize data from any sequencer.
For labs using an Illumina® sequencer and Illumina BaseSpace®, raw FASTQ sequencing files can be directly uploaded to QCI Secondary Analysis. For labs using other sequencers, a bulk file uploader tool can be used to upload data to QCI Secondary Analysis.
Once data has been uploaded to QCI Secondary Analysis, users then select a pre-configured analysis pipeline that has been optimized for their lab’s specific panel, policies, and standard operating procedures (SOPs). Users also have the option to run and deploy any bioinformatics tools, such as QIAGEN’s CLC Genomics Workbench, within QCI Secondary Analysis.
QCI Secondary Analysis automates the analysis of multiple sequencing runs simultaneously, generating high-quality variant calls within minutes. Users can then visualize the analysis results, such as read pileups, in a genome browser directly from the cloud platform. Or, users can send VCF files directly to QCI Interpret, or download the results to send to the interpretation platform of their choice.
QCI Secondary Analysis seamlessly integrates with QCI Interpret, QIAGEN’s platform for NGS variant interpretation and reporting, via QCI Connect, a secure, cloud-based application.
Through a preconfigured workflow for TruSight Oncology 500, QCI Interpret dynamically computes pathogenicity and actionability based on the AMP/ASCO/CAP or ACMG/AMP guidelines for every variant in over 31,000 cancer types with full transparency. To simplify and accelerate interpretation, users have access to over 320,000 preformulated, oncologist-reviewed variant impact summaries to build custom, patient-specific reports with the latest diagnostic and prognostic information, as well as biomarker-directed therapies and clinical trials.
With QCI Interpret, you can easily generate a final report with only the most relevant findings. While labs can customize the layout to fit branding and reporting needs, standard reports include:
Rare cancer variants are difficult to identify and interpret due to limited knowledge on their biological and clinical significance. In this application note, experts share a comprehensive genomic profiling workflow using the TruSight Oncology 500 assay and QCI Interpret to detect and analyze rare cancer variants.
Want to see what content QCI Interpret can provide for your variants? Please complete the form below and our experts will show you the depth of content that our software provides.