


















| Austria | Greece | Norway |
| Belgium | Iceland | Poland |
| Denmark | Ireland | Portugal |
| Finland | Italy | Spain |
| Germany | Netherlands | Türkiye |

Current users of QCI Interpret in Europe must transition to QCII IVD software to comply with the new In Vitro Diagnostic Regulation (IVDR). Please contact your sales representative to learn more about the transition.
QIAGEN Clinical Insight Interpret is a universal solution for tertiary analysis that can be used with any validated panel and sequencing platform. It provides variant annotation, classification, interpretation and reporting of somatic and germline NGS tests. The QCI Interpret product has been used to analyze and interpret over 4.5 million NGS patient test cases worldwide, making it one of the most widely used and universally respected platforms for efficiently accessing clinical evidence to support confident decision‑making in genetic testing.
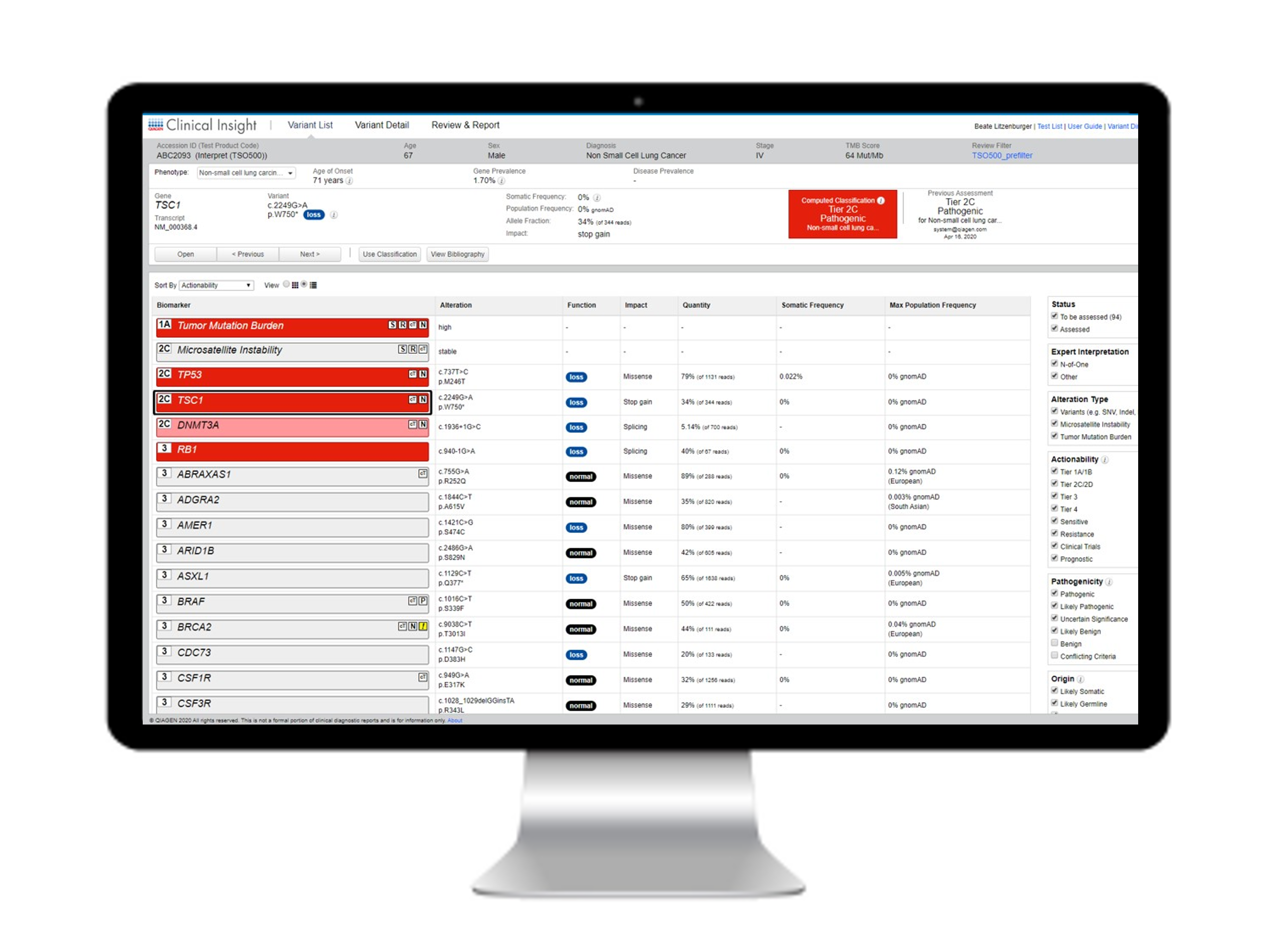
As a medical device software for clinical decision support (Class C IVD Medical Device), QCII IVD conforms with regulatory policy, ensuring greater patient protection, information transparency, and data traceability.
Unlike the EU, the Food and Drug Administration (FDA, North America), or other regulatory authorities (in different countries), have yet to take the next steps to regulate clinical decision support software that helps in analyzing and interpreting NGS variants.
The FDA is currently seeking community input and, with the final rule by FDA aimed at helping to ensure the safety and effectiveness of laboratory-developed tests (LDTs), it is becoming apparent that more and more IVD assays will become available (The ILLUMINA® TSO500 assay is in the process of IVD approval), which will require analysis and interpretation of NGS variants using a clinical decision support software that is compliant with an IVD assay quality management system. Essentially the FDA is expected to regulate the clinical decision support software, like the EU, in the future.
The fact that QCI Interpret is already IVDR approved, whenever the FDA decides to regulate clinical decision support software, QCI Interpret will readily be able to comply without any downtime, unlike other assays.
You can learn more about IVDR through the following resources:
Without additional effort for your laboratory, QCII IVD enables IVDR-compliant variant annotation and interpretation and simplifies clinical decision making.