The QCI Interpret Winter 2023 Release brings new variant assessment tools, functionality improvements, and expanded bibliographic coverage for carrier screening
We are pleased to announce that the Winter 2023 Release of QCI Interpret, QIAGEN’s decision support software platform for the annotation, classification, and reporting of somatic and germline variants, is now available.
Expanding on the software’s current capabilities, the QCI Interpret Winter 2023 Release brings new variant classification and assessment tools, functionality improvements, and even more variant content for prevalent hereditary diseases for faster, more informed variant analysis.
QCI Interpret Winter 2023 Release highlights
- New Triage Mode variant annotation tool in the Variant List page to accelerate variant review.
- Expanded bibliographic coverage for ACMG Tier 3 and Tier 4 carrier screening genes.
- New ClinVar column option added to the Variant List page so users have access to ClinVar’s summary of Variant Centric data to support variant interpretation within QCI Interpret.
- New Reportability status option added to enable the classification of a variant in an important edge case; users can now set a variant’s reportability to “Not Reportable – This Test Only” to handle this specific occurrence in a way that doesn’t affect the lab’s reporting policy.
- New variant-level “Verification Status” property, previously named “Validation”, added to allow users to collaboration on complex variants more efficiently with peers in a user group.
- New Settings page to allow users to customize and save default settings for how variant lists are organized and viewed.
- Improved sample management to enable users to attach ancillary documents, such as Quality Control reports.
- Improved test management tools with the addition of test flag icons to draw attention to specific tests and quickly annotate a case within the Test List page.
- Improved BAM/BAI resource handling to allow users to designate BAM (Binary sequence Alignment Mapping) file locations by Universal Resource Identifier (URI)
New Feature
Triage mode to streamline variant assessment workflows
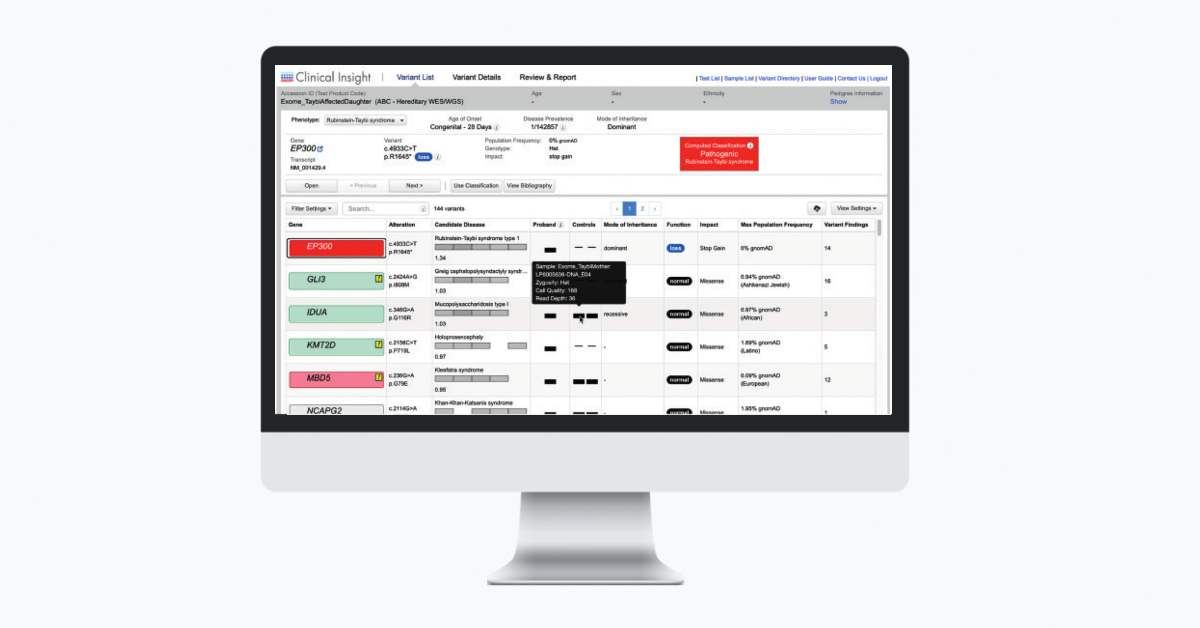
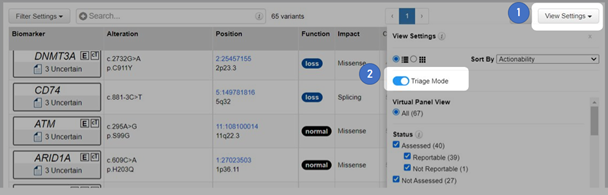
QCI Interpret now offers a new optional Triage Mode inline assessment tool to accelerate variant review workflows. The Triage Mode can be toggled on and off (Figure 1).
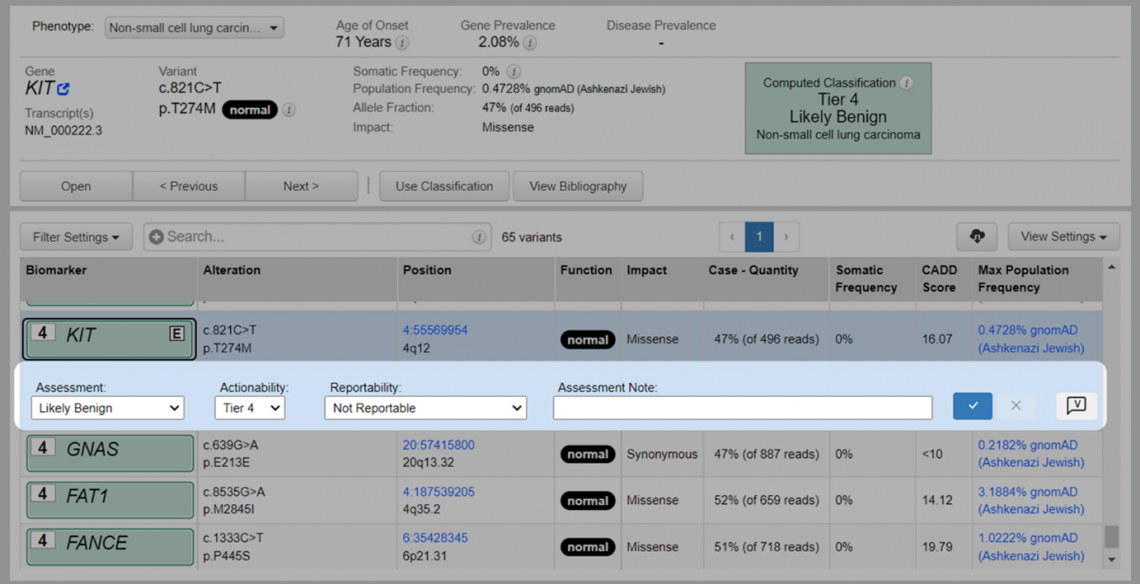
For novel unassessed variants, the Assessment, Actionability (somatic workflow only), and the Reportability values match the Computed Classification. Users can then add assessment notes (Figure 2). This new feature is intended to help high volume laboratories quickly assess and triage variants.
New Feature
QCI Interpret bibliography expansion for carrier screening
QCI Interpret’s literature coverage of genes, involved and associated with disease(s), strongly differentiates QCI Interpret from other decision support software by providing a fully certified and manually curated bibliography of approximately 1,000 genes with continuous expansion.
In the QCI Interpret Winter 2023 Release, the bibliography coverage is enhanced and expanded with a focus on genes that are routinely tested and proposed for carrier screening by the latest ACMG practice resource (Genetics in Medicine (2021) 23:1793–1806; https://doi.org/10.1038/s41436-021-01203-z).
Specifically, the bibliography coverage was fully certified and manually curated for Tier 3 genes with continued curation of Tier 4 genes, to provide a comprehensive and complete carrier screening interpretation workflow based on the latest ACMG recommendations summarized below:
- All pregnant patients and those planning a pregnancy should be offered Tier 3 carrier screening.
- Tier 4 screening should be considered: (1) When a pregnancy stems from a known or possible consanguineous relationship (second cousins or closer). (2) When a family or personal medical history warrants.
New Feature
New ClinVar column on the Variant List’s Variant table
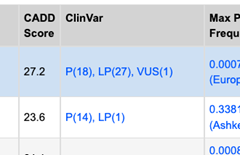
In QCI Interpret, a new ClinVar column displays the assessment (P, LP, VUS, LB, B) and number of ClinVar submitters as pulled from the QIAGEN Knowledge Base. A hyperlink takes the user to the respective ClinVar VCV page (phenotype agnostic) (Figure 3).
Pathogenic (P), Likely Pathogenic (LP), VUS, Likely Benign (LB), and Benign (B) interpretations sent to ClinVar are tallied. The hyperlink takes the user to the NCBI variant specific page for additional detail.
For the complete QCI Interpret Winter 2023 Release Notes, please contact your QIAGEN Digital Insights account representative or email our support team at ts-bioinformatics@qiagen.com.
About QCI Interpret
QCI Interpret is a clinical decision support software platform for the annotation, classification, and reporting of actionable alterations from NGS data for oncology and hereditary disease applications. Using augmented molecular intelligence and expertly curated content from the QIAGEN Knowledge Base, QCI Interpret applies a rules-based approach to automatically compute pathogenicity classifications (Pathogenic to Benign) and actionability classifications (Tier 1 to 4) for each alteration according to professional guidelines from ACMG/AMP and AMP/ASCO/CAP, respectively.
Pathogenicity and actionability classifications in QCI Interpret are accompanied by clear visibility into the criteria and evidence supporting the classifications. This workflow starts with a variant call format (VCF) file, so it is compatible with the output from any NGS platform. The final report includes the alterations, interpretations, and references specified throughout the assessment process, which has customizable automation capabilities allowing for streamlined clinical decision support workflows.