
Oncology
Provide oncologists with patient-specific diagnostic and prognostic reports for any alteration in any cancer type with clinical decision support software or professional interpretation services
Home > Clinical Research Solutions > Oncology Testing Solutions
17 million
new cancer cases diagnosed every year (1)
85%
of the global oncology market is focused on targeted therapies (2)
100+
new clinical trials open each month (3)
Reduce time to interpret clinical NGS tests by 85%
Nothing about cancer is simple. With such a life-changing diagnosis, oncologists need confidence that their genetic testing labs are considering the most up-to-date, relevant data to guide their diagnostic and treatment decisions.
QIAGEN Clinical Insights (QCI) for Oncology offers software and services to empower molecular pathologists with expert-curated content to intelligently navigate the biological characteristics of genetic alterations and their clinical relevance to a patient’s tumor profile. Capable of interpreting any alteration in any cancer type, QCI for Oncology helps molecular pathologists deliver personalized, evidence-based treatment recommendations for an expanding menu of tests in a fraction of the time.
In-sourced or out-sourced?
Whether building a first-class NGS variant interpretation and reporting pipeline or looking for an experienced partner to interpret tests for you, QCI for Oncology has two trusted solutions to fit your lab’s specific needs:
Application Note
Developing a robust, automated, and streamlined clinical NGS workflow for hematological malignancies
In this application note, we discuss the importance of streamlined clinical NGS workflows within the hematologic-oncology space. Learn how to develop a robust, automated, and streamlined NGS analysis pipeline for the interpretation and reporting of genomic alterations associated with hematological malignancies.
“Why Precision Insights? We save time. In clinical practice, outside of academics, most people don’t have time to do the research at an adequate level and with consistency required from one case to the next. We get an 85-90% reduction in case preparation time using Precision Insights.”
Anne Berry, M.D.,
Scientific Director of Personalized Medicine and Medical Director of Molecular Diagnostics
Swedish Cancer Institute and CellNetix Pathology and Laboratories
Provide actionable cancer care insights
With the promise of precision medicine becoming a reality, molecular profiling has become standard of care for many cancer types. More than ever, oncologists need a trusted profiling partner to provide reliable, high-quality molecular information to guide more precise and individualized treatment decisions.
QCI for Oncology enables standardized reporting according to AMP/ASCO/CAP guidelines for the interpretation and reporting of somatic variants (4) and provides biological insights based on ACMG guidelines (5).
To ensure consistency and reproducibility of results, QCI for Oncology provides full transparency to review the criteria and evidence supporting each classification and gives you real-time visibility into active clinical trial registries and available FDA-approved therapeutics.
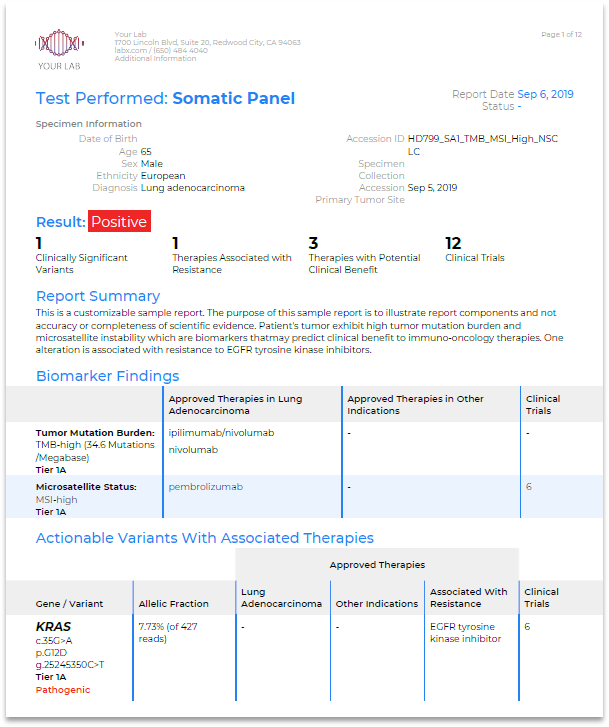
Clinical oncology testing solutions

QCI Interpret
For molecular diagnostic labs performing variant interpretation and reporting in-house, QCI Interpret enables faster test turnaround times and higher confidence reporting for any assay on your sequencing platform.

QCI Precision Insights
For molecular diagnostic labs outsourcing somatic variant interpretation, QCI Precision Insights, powered by N-of-One, offers rapid turnaround times and customized variant-level interpretation for patient-specific reports.
QCI Interpret One
Clinical decision support software integrated with professional variant interpretation services that enables rapid, evidence-based reporting for NGS oncology testing at scale.

Interpreting NGS cancer panels?
Whether new to NGS or an experienced user, learn how QCI for Oncology can increase your lab’s efficiency, confidence, and test menu offerings.
Speak with a clinical testing expert today.
Related clinical testing solutions

Hereditary Diseases
Empower clinicians and their patients to make critical and timely healthcare decisions with the latest publications and clinical evidence

QIAGEN Clinical Insights
Deliver patient-specific reports for any NGS panel in minutes with on-demand, expert-curated content and professional interpretation services

Clinical Analysis and Interpretation Services
Leverage the benefits of automation and expert support to improve test turnaround times and clinical reporting capabilities