While AI can analyze massive data sets, it lacks the human insight needed for complex, nuanced decisions. In clinical genetic testing, accuracy is everything—and expert curation remains the gold standard, ensuring reliable, context-driven interpretations.
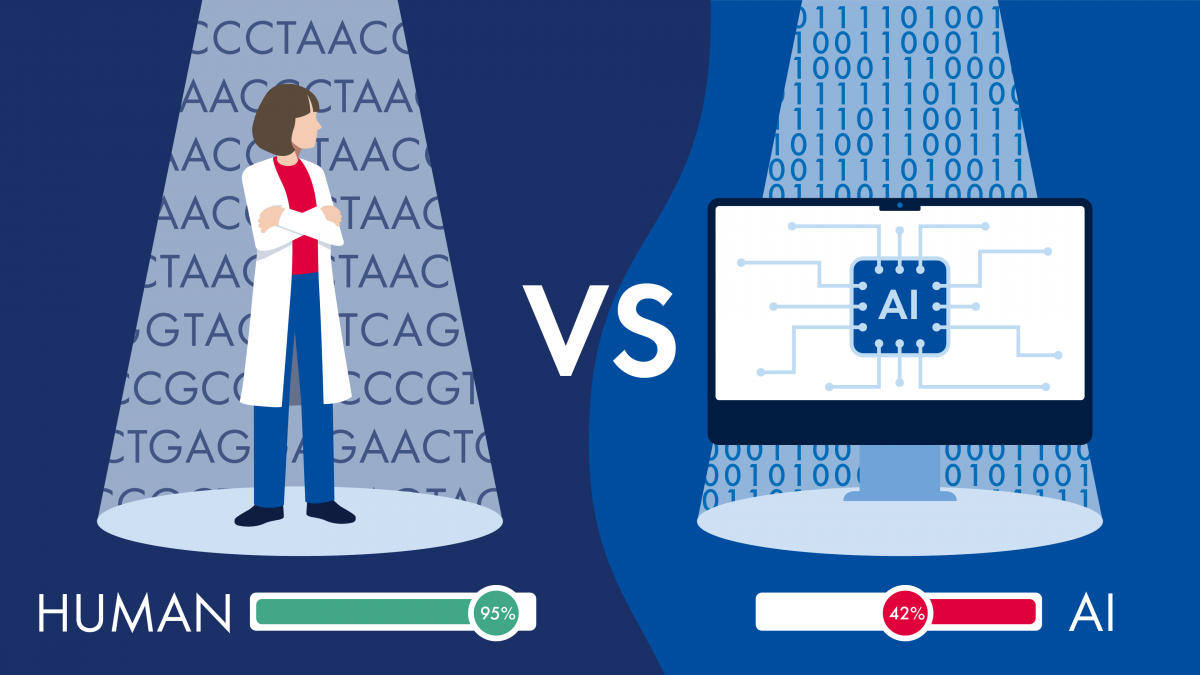
As genetic testing continues to play an increasingly pivotal role in personalized medicine, one question has become central for clinical genetic testing labs: should they rely on AI or expert curation for variant classification? Each approach offers distinct advantages and challenges, and understanding the strengths of both is key to delivering accurate, actionable results for patients.
The role of variant classification in genetic testing
For years, variant interpretation has been carried out by highly skilled human experts, such as geneticists and bioinformaticians, who manually review scientific literature, databases, and other resources to determine whether a variant is benign, pathogenic, or of uncertain significance. However, with the explosive growth of genetic data, manual curation alone has become a bottleneck, prompting the development of AI-driven solutions. But can clinical genetic testing labs trust AI to deliver accurate results? Or is expert curation still the gold-standard method of variant curation?
Below, we analyze the strengths and weaknesses of both approaches and present the findings of an independent study conducted by the National Institutes of Health (NIH), in which investigators compare the clinical utility of four popular literature mining tools for variant curation.
The case for AI in variant curation
AI offers a powerful, scalable solution to the volume problem. But while AI delivers speed and efficiency, the approach has its limitations.
Strengths of AI
- Speed and efficiency: AI can sift through vast amounts of data—including scientific publications, databases, and variant repositories—at speeds no human can match. AI tools such as machine learning algorithms can quickly identify patterns and make initial classifications, significantly reducing the time needed to analyze large datasets.
- Data mining at scale: AI systems can scan enormous data repositories, such as public variant databases (e.g., ClinVar) or scientific literature, to identify correlations between variants and clinical outcomes. However, AI systems are only as good as the data they access.
- Continuous learning: As AI models are exposed to more data, they can improve over time. Machine learning algorithms can adapt and become more accurate as new data and outcomes are fed into the system, helping to refine predictions. But, while AI may become more accurate in the future, can it be confidently applied to clinical applications today?
Limitations of AI
- Lack of contextual understanding: AI models primarily rely on statistical correlations and data patterns. However, the clinical significance of genetic variants often depends on complex contextual factors such as patient phenotype, inheritance patterns, and environmental influences. AI struggles to understand and integrate this level of biological and clinical nuance, which human experts can better interpret.
- Data quality and incomplete datasets: AI tools are only as good as the data they are trained on. Public genetic variant databases, such as ClinVar or other literature sources, may contain incomplete, conflicting, or outdated information. AI models, lacking human judgment, can misclassify variants if they rely on low-quality or inaccurate data sources without filtering them for relevance or reliability.
- Inability to handle ambiguous or novel variants: One of the major challenges in clinical genetics is interpreting variants of uncertain significance (VUS). AI models may struggle to classify these variants accurately because they rely on existing patterns in data, and novel or rare variants might not have sufficient supporting evidence for the AI to draw reliable conclusions. Expert curators can apply clinical expertise and scientific reasoning in ways AI currently cannot.
The case for expert curation
Despite the advantages of AI, expert curation remains crucial for accurate variant interpretation. Here’s why human expertise is still irreplaceable:
Strengths of expert curation
- Complex contextual interpretation: Genetic variants do not exist in isolation. Many times, their significance depends on factors like gene function, inheritance patterns, or clinical presentation. Expert curators have the ability to synthesize these contextual details, taking into account not only the raw data but also the broader clinical and biological significance of a variant.
- Quality over quantity: AI may be able to process data quickly, but human curators can scrutinize the quality and relevance of the sources it uses. Experts can critically evaluate scientific papers, distinguishing well-supported findings from preliminary or low-confidence studies. This ensures that only reliable, high-quality information is used in final classifications.
- Nuanced decision-making: Certain genetic variants are challenging to classify due to incomplete or conflicting evidence. In such cases, human curators are better equipped to make nuanced judgments, applying their professional knowledge and experience to interpret ambiguous or borderline cases.
Limitations of expert curation
- Limited scalability: Human expertise doesn’t easily scale. As the demand for genetic testing rises, the number of qualified variant curators may not keep pace with the volume of data being generated. However, this is where an expert-curated database, like the Human Gene Mutation Database (HGMD) Professional, can prove advantageous. For example, HGMD Professional curators use machine-learning approaches to massively index and identify relevant literature sources. Then, highly trained expert curators review each piece of evidence to ensure accuracy, consistency, and context.
- Resource intensive: Training, employing, and retaining highly specialized curators require significant resources. Expert curation teams often need ongoing professional development to stay current with evolving guidelines, new research, and updated variant classification criteria. Smaller labs, in particular, may find it difficult to maintain the necessary infrastructure to support expert-driven curation. This is again where using an expert-curated database like HGMD Professional can help overcome resource limitations. The HGMD Professional curation team does the heavy lifting, so you don’t have to.
NIH study finds expert curation demonstrates a 126% higher precision score than AI-derived database
In Genetics in Medicine, a study entitled, “Comparison of literature mining tools for variant classification: Through the lens of 50 RYR1 variants” was published by Wermers et al. of the Center for Precision Health Research at the NIH. Recognizing the importance of accessing relevant literature that informs the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) criteria for classifying variant pathogenicity, investigators set out to determine the efficacy of four literature mining tools in the retrieval of publications to classify 50 RYR1-related malignant hyperthermia susceptibility genes. The four tools included QIAGEN’s HGMD Professional, Genomenon® Mastermind®, ClinVar, and LitVar2.
Want to learn more about HGMD Professional?
The advantages of using HGMD Professional is that it offers comprehensive coverage (on over 520,000 germline mutations that have been reported in the literature), expert curation, quarterly updates, and clinical utility (providing information on the clinical significance of germline mutations, including disease association, inheritance patterns, and pathogenicity).
To demonstrate the quality of utility of HGMD Professional, QIAGEN Digital Insights offers free, no-obligation 5-day trials of the licensed database.