Peer-reviewed study compares accuracy and consistency of variant assessments from commercial CDS software to the internal variant interpretation methods of 8 laboratories
Over the last 20 years, molecular analysis of cancers has offered clinicians a growing toolbox for understanding and treating cancer. Next-generation sequencing (NGS) of tumors identifies alterations that can predict sensitivity and resistance to targeted therapies as well as ascribe prognostic and diagnostic significance. As sequencing power and research into cancer-causing mutations have grown, the number of genes on panels has increased.
In 2022, typical panels can detect hundreds of thousands of mutations across several hundred cancer-related genes. In some cases, laboratories will perform exome analysis with the power to detect mutations across all ~22,000 genes in the human genome. As a result, the burden of variant interpretation has also expanded exponentially.
Clinical decision support systems
Numerous clinical decision support systems and knowledgebases have been developed to assist variant scientists and laboratory directors with the task of variant classification. These private and commercially available systems utilize varying degrees of software automation and manually curated literature to provide variant assessment and therapy matching for clinicians. The body of literature that must be accessed to deliver accurate variant interpretation is vast and there is debate in the field as to the most accurate and efficient approach.
Artificial intelligence or natural language processing can index enormous volumes of literature but lacks precision in correctly representing complex genomic interactions in association with clinical outcomes; in this context, human curation remains the gold standard. A community crowdsourcing approach allows contribution from many different experts and can help to build a larger pool of knowledge in the context of limited resources.
However, significant standardization efforts are required to ensure a consistent level of accuracy and reliability. In contrast to machine curation, human professional expert curation is resource intensive but can provide consistent and accurate interpretation.
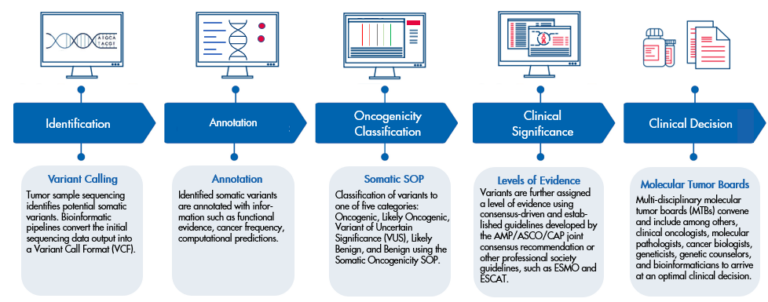
Sample workflow of how clinical decision support software aids somatic variant interpretation
QIAGEN Clinical Insight (QCI) Interpret for Oncology is clinical decision support software that enables pathologists to identify biologically and clinically relevant oncology-related variants. The software draws
on a large knowledgebase of curated information, coupled with an expert interpretation service. The content core of QCI Interpret, the QIAGEN Knowledge Base is populated through a combined approach utilizing human and machine curation. Known as augmented molecular intelligence (AMI), this approach combines artificial intelligence and human expertise to advance and accelerate confident clinical decision-making.
What is augmented molecular intelligence?
A key differentiator of QCI Interpret, the application of AMI leverages artificial intelligence and machine
learning to efficiently identify, extract, and align evidence from scientific literature, guidelines, clinical trials, and drug labels in over 40 public and proprietary databases in the QIAGEN Knowledge Base. This
evidence is then reviewed by over 200 PhD- and MDlevel scientists to ensure accuracy, consistency, and
relevance. The evidence is then stored in computable units according to well-defined protocols.
How QCI Interpret classifies variants
QCI Interpret utilizes the structured content of the QIAGEN Knowledge Base to match appropriate variant- and disease-specific content and executes rules to classify variant pathogenicity and actionability based on the ACMG (1) and AMP (2) guidelines. The computed classification and supporting data are available for review in a user interface, and the user has the ability to review all the data and approve or revise the classification.
QCI Interpret also incorporates an additional level of human expert interpretation. The variants are submitted in the context of the disease to the expert interpretation service, staffed by trained professional expert scientists with clinical content reviewed by oncologists.
The expert interpretation available in QCI Interpret utilizes a contrasting analysis approach; the scientists execute a topic-based analysis, searching for and extracting information on each variant and formulating an assessment based on the synthesis of the evidence. The expert interpretation supplies report-ready text with references that can be reviewed and incorporated into clinical reports. The expert classification and interpretation are presented in a user interface alongside the computed classification and the user has another opportunity to review the summarized data and approve or revise the classification for reporting.
Assessing the accuracy of QCI Interpret
Multiple studies have been published comparing variant classification across institutions and performance of different variant interpretation and clinical decision support software (3-5). However, all of these studies lack a “gold standard” set of variant interpretations that could stand as a benchmark for evaluation.
In order to assess the utility and accuracy of QCI Interpret, QIAGEN engaged GenQA, an external quality assessment organization, to design and execute a study that would compare the use of QCI Interpret to internal laboratory methods. The study involved the recruitment of eight independent laboratories to utilize QCI Interpret for variant interpretation, compared the results to the originally reported classifications and employed an expert panel to resolve conflicts. The results suggest that QCI Interpret is a reliable clinical decision support tool that can help laboratories streamline and improve their interpretation practices.
Download a free copy of the study published in the Journal of Molecular Pathology.
Learn more about the study, including how QCI Interpret performed against the 8 laboratories, sources of discrepancies, and methods of variant analysis.
Meet us at AMP 2022
November 1-5, 2022 in Phoenix, Arizona
Booth #906
This year at the Association for Molecular Pathology (AMP) 2022 Annual Meeting, QIAGEN will be showcasing our integrated cancer NGS workflow powered by augmented molecular intelligence (AMI) at Booth #906. The combination of artificial intelligence and human expertise, AMI is an approach unique to QIAGEN that uses machines to rapidly index millions of articles and human curators to review and certify the accuracy, relevancy, and consistency of the information pulled.
Learn more and schedule a 1:1 demo here.

