
QIAGEN Clinical Insights
Deliver patient-specific reports for any NGS panel in minutes with on-demand, expert-curated content and professional interpretation services
Home > Bioinformatics Products Overview > Clinical Research Databases & Insights Portfolio > QIAGEN Clinical Interpretation Services
1 million
medical papers are added to data sources like PubMed each year
100
clinical trial reports and reviews are added every day†
1,000+
new cancer drugs were being clinically studied or awaiting FDA review in 2018*
Uncover critical and timely genomics insights
If genomic data is the currency of modern medicine, there’s a premium on clinical insight.
Today’s health care industry is witnessing a paradigm shift that is driving a tighter integration of genomic analysis modalities in patient care decisions. From disease diagnostics and prognostics to carrier screening and clinical trial enrollment, physicians and oncologists are ordering a growing number of genetic tests for an expanding menu of applications.
QIAGEN Clinical Insights (QCI) offers expert-curated knowledge, software and services to support clinical NGS data analysis and interpretation for any indication, on your platform, in a fraction of the time.
Clinical testing applications:
Having access to the most comprehensive and up-to-date catalog of known mutations augments our existing variant classification expertise. This will allow us to continue to provide physicians and researchers with the best possible test interpretations, advancing LabCorp’s mission to improve health and improve lives.
Marcia Eisenberg, Ph.D.,
Chief Scientific Officer for LabCorp Diagnostics
Stay connected to the evidence you need
QIAGEN maintains the world’s largest, expert-curated knowledgebase of up-to-date evidence for clinical test interpretation. For over 20 years, we have been reading, collecting, and ontologically organizing data from the latest publications, guidelines, FDA therapeutics, and clinical trials, to deliver time-saving insight.
More than a data repository, the QIAGEN Knowledge Base refreshes weekly with the evidence you need.
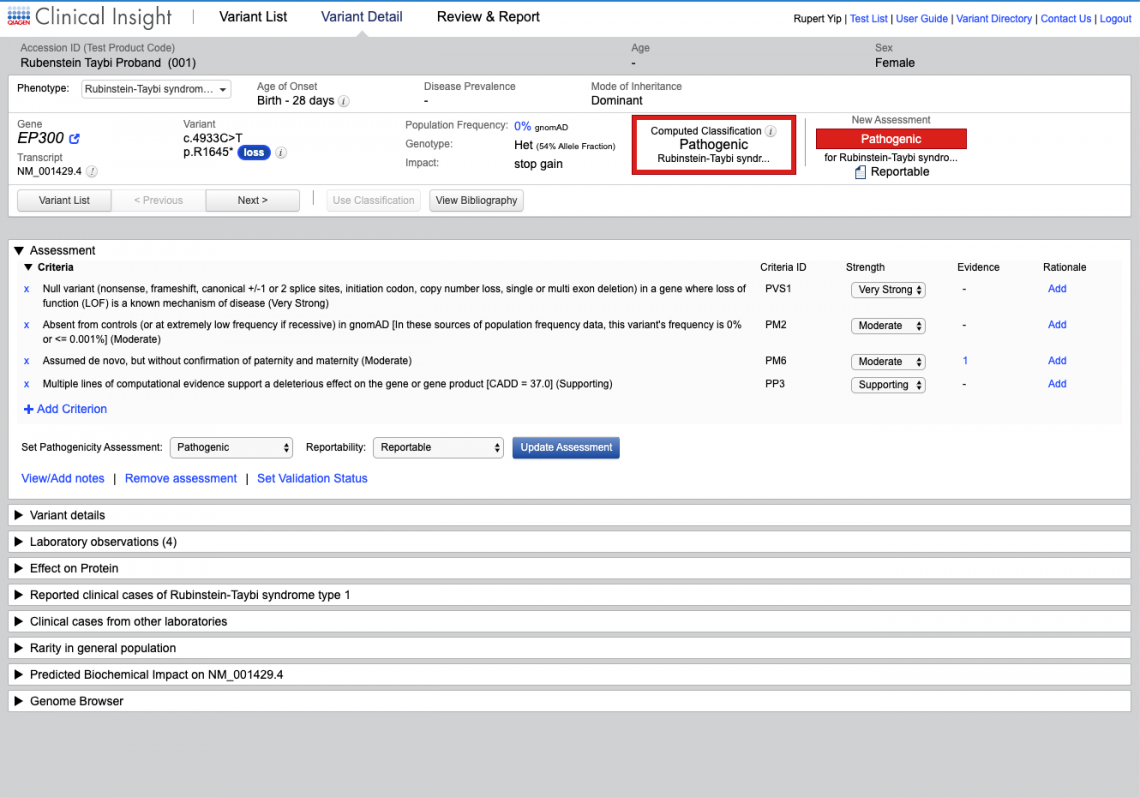
QCI products and services

QCI Interpret
For molecular diagnostic labs performing variant interpretation and reporting in-house, QCI Interpret enables faster test turnaround times and higher confidence reporting for any assay on your sequencing platform.

QCI Precision Insights
For molecular diagnostic labs outsourcing somatic variant interpretation, QCI Precision Insights, powered by N-of-One, offers rapid turnaround times and customized variant-level interpretation for patient-specific reports.
QCI Interpret One
Clinical decision support software integrated with professional variant interpretation services that enables rapid, evidence-based reporting for NGS oncology testing at scale.
QIAGEN Knowledge Base
The information core of QCI, the QIAGEN Knowledge Base is the industry’s most expansive collection of up-to-date biological and clinical genomic findings, spanning across 40 public and proprietary databases curated over two decades by certified content curation experts.

Interested in QCI?
Speak with a clinical testing expert today to learn more about QCI and which interpretation solution is right for your lab
Related clinical testing solutions

Hereditary Diseases
Empower clinicians and their patients to make critical and timely healthcare decisions with the latest publications and clinical evidence

Clinical Analysis and Interpretation Services
Leverage the benefits of automation and expert support to improve test turnaround times and clinical reporting capabilities
*Landhuis, E. Scientific Literature: Information Overload. Nature. 2016 July 21;7612:457-58.
†Statista. The Statistics Portal: Number of cancer drugs in development in the United States in select years between 2005 and 2018. Pharmaceuticals Products & Market.
QIAGEN Clinical Insight (QCI) is an evidence-based decision support software intended as an aid in the interpretation of variants observed in genomic sequencing data. The software evaluates genomic variants in the context of published biomedical literature, professional association guidelines, publicly available databases and annotations, drug labels, and clinical-trials. Based on this evaluation, the software proposes a classification to aid in the interpretation of observed variants. The software is NOT intended as a primary diagnostic tool by physicians or to be used as a substitute for professional healthcare advice. Each laboratory is responsible for ensuring compliance with applicable international, national, and local clinical laboratory regulations and other specific accreditations requirements.
